The FDA's quality system requirements for all medical device companies are documented in the federal code of regulations, under 21 CFR Part 820.
Under federal regulations established by the FDA, 21 CFR, medical device manufacturers in the United States are required to establish and maintain a quality system for ensuring the safety and effectiveness of their medical devices for the end user. Quality systems are a set of policies and procedures that ensure quality and safety throughout the manufacturing process.
Greenlight Guru's QMS software is the ideal companion software for medical device companies seeking to comply with 21 CFR Part 820.
Overview Of 21 CFR Part 820 Requirements
FDA mandates, under 21 CFR Part 820, that each medical device company must establish and maintain a quality system that meets the requirements of its regulations and is appropriate for the medical device they manufacture. Differently classified medical devices may have different requirements under 21 CFR Part 820. For example, Class I medical devices (with some exceptions) are exempt from the design controls portion of the regulations, but in general, the guidelines must be satisfied to permit the sale of a medical device within the United States.
Medical device companies should consider the quality system regulations, such as 21 CFR Part 820, as their "key to admission" into
QMS Enables And Supports Compliance With 21 CFR Part 820
Quality management software (QMS) enables medical device companies to effectively monitor and manage their compliance efforts in regards to 21 CFR Part 820, ensuring a smooth FDA audit process, successful 510(k) applications and premarket approval, and a shortened time to market. Here are some of the challenges presented by 21 CFR Part 820 and how Greenlight Guru can help your company manage them with its cutting-edge QMS software.
Management Controls (21 CFR Part 820.20)
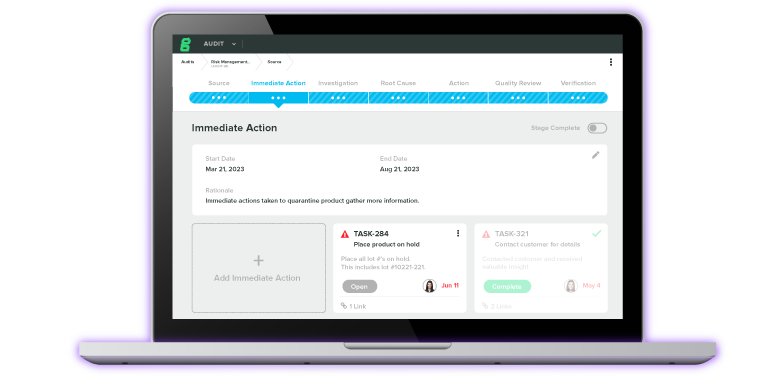
21 CFR Part 820 requires that medical device companies designate a quality manager whose role is to oversee the quality system. A quality policy, management review and quality audit procedures, quality plan and quality system procedures and instructions should all be defined, documented, and implemented throughout the organization. The appointed manager must be conducting regular reviews of the effectiveness of the system, along with internal quality audits and re-audits of deficient matters.
Greenlight Guru's QMS Software centralizes all organizational quality data in one system where a quality manager can easily access and reference the materials necessary to perform their role. A searchable, organized database is an asset for quality managers, whose role requires them to view and review the hundreds of documents generated by your company's quality process.
Document Controls (21 CFR Part 820.40)
21 CFR Part 820 includes a mandate for document control - manufacturers must designate someone to review and approve all documentation generated by the quality system. Any documents produced need to be available everywhere they are designated and obsolete documents should be removed from use immediately.
Greenlight Guru's QMS Software includes built-in document controls, e-signatures, and document history. Updated documents are automatically replaced by their newest version, and the designated individual can review and approve new documents from the comfort of their desk, instead of chasing department managers around for the latest paperwork and spending hours organizing a paper filing system. Learn more about document management here.
Design Controls (21 CFR Part 820.30)
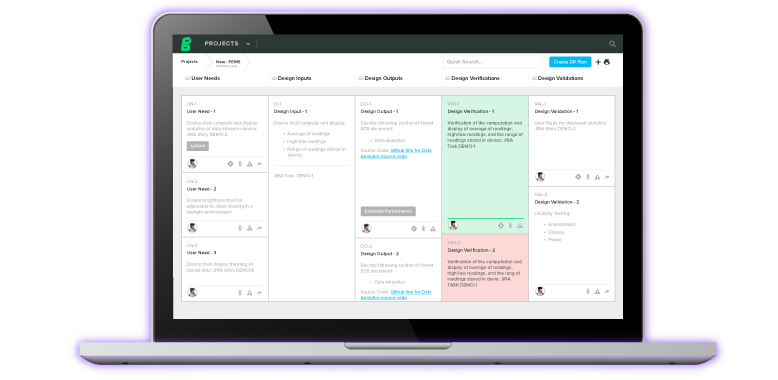
Design controls are one of the major processes mandated in 21 CFR Part 820. This mandate under 21 CFR requires manufacturers to establish and maintain procedures that control the design of the medical device, helping to ensure that it meets the specified design requirements. Identifying and documenting user needs, and design input and output, conducting design reviews, verification and validation, and properly implementing design transfer and changes are all part of this process.
Greenlight Guru's QMS Software is the ideal support tool for medical device development, including the establishment and documentation of design controls. Greenlight Guru helps ensure that no step is missed along the pathway to compliance with 21 CFR Part 820 and that your company produces quality documentation that feeds into your design history file (DHF) and device master record (DMR), helping you achieve compliance and accelerating your time to market. We've also created the Ultimate Guide to Design Controls to help you get started.








%20Ultimate%20Guide%20to%2021%20CFR%20Part%20820%20%E2%80%94%20FDA%20Quality%20System%20Regulation%20(QSR)%20for%20Medical%20Devices.png?width=5100&name=(cover)%20Ultimate%20Guide%20to%2021%20CFR%20Part%20820%20%E2%80%94%20FDA%20Quality%20System%20Regulation%20(QSR)%20for%20Medical%20Devices.png)