Beginner's Guide to Design Verification & Design Validation for Medical Devices

Design verification and design validation are two essential stages in design controls. It’s easy to confuse the two because they both involve checking an outcome against your previous specifications.
That and, well, they’re both long words that start with ‘V’.
But not to worry—in this guide, we’ll go through the basics of design verification and design validation, best practices you should follow, and common problems to avoid.
What's the difference between design verification and design validation?
Here’s the simplest way to remember the difference between design verification and design validation:
-
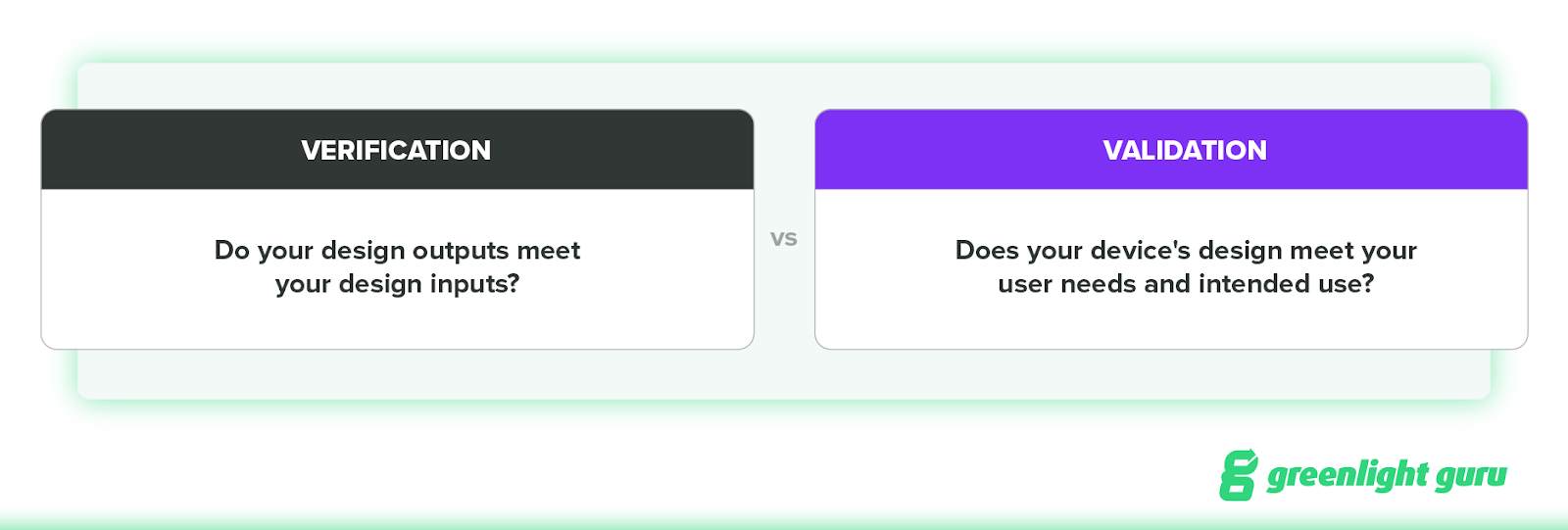
During design verification, you verify that your design outputs meet your design inputs. In other words, did you design the device right?
-
During design validation, you prove that the device’s design meets the user needs and intended uses you’ve specified. In other words, did you design the right device?
For a good visual representation of this dynamic, simply look at the FDA’s design controls waterfall diagram.
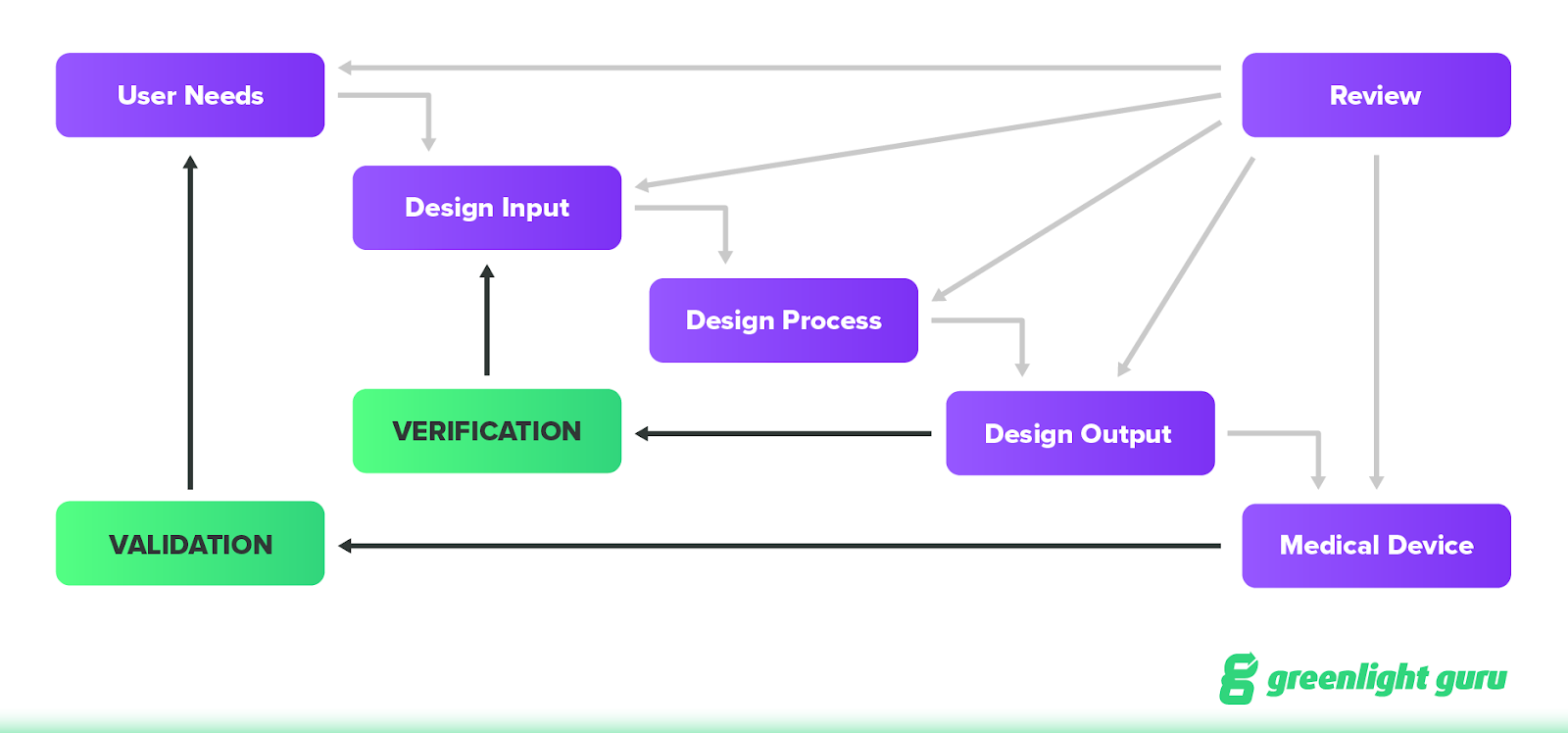
Remember, user needs inform design inputs, but they’re not the same thing. Your design inputs are intended to capture all functional, performance, safety, and regulatory requirements in a way that builds upon your user requirements.
For instance, one of your user needs may be that the user must be capable of operating the device with one hand. But that single user need may generate several design inputs related to size, weight, and ergonomics, just to name a few.
In this example, design verification checks that your design outputs—such as drawings, specifications, or manufacturing instructions—meet those design inputs.
Design validation, on the other hand, examines your user needs. So, in this example, one of the user needs you’d need to validate would be one-handed operation. Can the device be operated with one hand and fulfill its intended use?
While these may seem like very similar processes, you absolutely must go through both design verification and validation. It’s entirely possible to have your design outputs meet your design inputs perfectly—and end up with a device that doesn’t meet your user’s needs.
Now that we’re clear on the differences between the two, let’s take a look at how you should carry out design verification and design validation.
What are design verification best practices?
In 21 CFR Part 820.3, FDA states that, “Verification means confirmation by examination and provision of objective evidence that specified requirements have been fulfilled.” In other words, do your design outputs meet your design inputs (your “specified requirements”)?
More often than not, design verification involves suites of tests and trials. However, there are other acceptable verification activities, such as inspection and analysis (for a more extensive list of activities, refer to page 30 of the FDA Design Control Guidance).
Whatever the case, your design verification activities are intended to prove that you’ve met the requirements of your design inputs. And that’s why the foundation of an effective verification testing process is well-defined design inputs.
There are a few things to remember when writing your design inputs:
-
Ask yourself what your device needs to do and what it needs to go through to achieve its intended purpose. If you’re designing a catheter, for instance, you might consider how much liquid it needs to move and at what speed.
-
Define what conditions are best for your device and how those might change. Your device may be intended for use in a hospital room, but it may also need to accommodate a certain amount of movement. What if the patient has to be moved to a new room or the OR?
-
Make these design inputs as clear, discrete, and actionable as possible. Ambiguity leads to mistakes—and mistakes require rework. Rework is expensive, slows down the product development process, and can demoralize your team.
-
Write design inputs that are testable. As you write your design inputs, you need to consider whether they can actually be verified later on. What tests or analysis will you perform to demonstrate that your design outputs meet your design inputs?
By the end of the process, you should have a list of design inputs and verification tests for each that will demonstrate that the device does what you intend it to do.
What are design validation best practices?
The purpose of design validation is to prove you designed the right device. Doing so means proving the medical device meets the user needs and intended uses. Or, as FDA puts it: “Validation means confirmation by examination and provision of objective evidence that the particular requirements for a specific intended use can be consistently fulfilled.”
There are several best practices nearly every design validation process must involve:
-
Your design validation process must include initial production units. This means the medical devices used for validation have to be built in the production environment, using drawings and specifications (i.e., design outputs) by production personnel.
-
Design validation must involve clinical evaluation. This means that the end-user(s) should be involved, and the device should be tested either under simulated use or actual use. Simulated validation often includes mathematical modeling. You’ll want to compare your device against others with similar purposes.
-
Use the medical device under the specific, intended environmental conditions. This includes any changing conditions, such as devices that must remain operable as patients move from room to room.
-
Don’t exclude the packaging and labeling. Your medical device isn’t just the hardware. A medical device includes everything from the label, the instructions for use, the packaging, and everything inside your packaging. Validation must address all of it.
Creating effective design verification and validation plans
Plans are what separate your process from chaos. If you don’t have plans in place for verification and validation, you’ll end up wasting an enormous amount of time on these steps, and you may miss key activities that could hold up your regulatory timeline.
The most important thing to remember when it comes to planning design verification and validation activities is this: start early.
Design validation happens late in product development, but it’s based on the first step in the design controls process—defining your user needs. And as soon as you have your user needs, you can begin planning how you’ll validate them later on. There’s no need to wait until you reach the validation step to start thinking about it.
That goes for design verification, too. The sooner you begin thinking about how you’ll verify these design inputs, the smoother the actual verification process will go. In fact, you should be considering how you’ll verify your design inputs while you’re defining them.
Rushing to the design verification and validation stages doesn’t actually save you time. It just ensures you’ll spend even more time and energy trying to verify unclear design inputs or validate the poorly defined user needs.
Incorporating design changes after your initial verification and validation
At some point, you will need to update your product.
This could happen before you even put your device on the market. Maybe testing during the verification and validation stages reveal issues that can only be fixed with a change to the design.
But post-market information, such as nonconformances, customer feedback, or corrective and preventive actions (CAPA) might necessitate a change to the design after your product is on the market.
Regardless of when you decide to update the product, the process for making that change is the same. It’s a familiar process because it resembles what you did during design and development.
With your new information in hand, from pre- or post-product launch, start reviewing. Go back through all your user needs, design inputs, and design outputs. Update everything in light of the new information you have.
After a thorough review, it’s time to start verification and validation again. No matter when in the product lifecycle you make the change, you can’t skip verification and validation. Even in mid- and post-market stages, before and after product updates, you have to be ready to verify and validate.
That doesn’t mean you’re starting from scratch every time. You can always leverage previous lessons, experiences, and data, and the types of design changes you’ve made will determine how much verification and validation you’ll need to redo. But there simply can’t be a point in the process where you’re unsure whether you’ve made the right device and that it’s working correctly. That means verifying and validating—every time.
Keep all your design control activities in one centralized location with Greenlight Guru
Design verification and validation don’t happen in a vacuum. They’re steps in your design controls that are intimately connected to the rest of product development. And making sure those connections are clear and fully traceable is a fundamental requirement for any medical device company’s QMS.
That’s why at Greenlight Guru, our QMS platform allows you to create detailed design control objects, link complex configurations, and attach documents with a single click. You’ll be able to easily navigate between test reports and protocols to your design control verifications and validations, while also seeing related design inputs, risk controls, and components.
So if you’re ready to see what a purpose-built QMS can do for your medical device company, then get your free demo of Greenlight Guru today!
Looking for a design control solution to help you bring safer medical devices to market faster with less risk? Click here to take a quick tour of Greenlight Guru's Medical Device QMS software →
Jesseca Lyons is a Senior Medical Device Guru at Greenlight Guru and a Mechanical Engineer by trade who loves working with cross functional teams, including both engineering and non-engineering disciplines. She’s spent most of her career gathering and defining requirements for new product design and development in the...
Related Posts
How Priority Designs Used Greenlight Guru to Gain ISO 13485:2016 Certification
[VIDEO] Integrating Design Controls & Risk Management To Streamline Product Development (Make Phase)
How to Define and Decode Your Design Inputs and Design Outputs
Get your free eBook PDF
The Beginner's Guide to Design Verification and Design Validation for Medical Devices
%20Design%20Verification%20%26%20Validation.png?width=250&name=(cover)%20Design%20Verification%20%26%20Validation.png)







%20Design%20Verification%20%26%20Validation.png?width=1700&name=(cover)%20Design%20Verification%20%26%20Validation.png)