
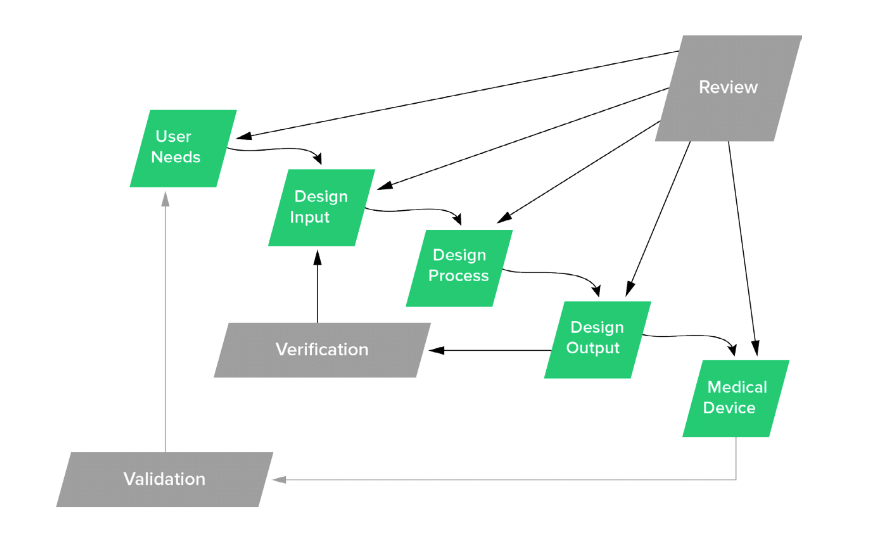
In the FDA’s classic design control waterfall diagram, the box at the left-hand top of the waterfall is labeled “user needs.”
Surprisingly, though, I’ve seen many cases throughout my career where the product development team failed to formally capture and document user needs. Most of these teams were aware of user needs—they just didn’t take the time to document them.
Instead, they assumed that they had a clear understanding of the user needs in their heads as they proceeded through product development.
This is playing with fire. And to understand why, let’s walk through how medical device companies should define user needs and incorporate them into the design control process.
FREE RESOURCE: Click here to download the free eBook of the Ultimate Guide to Design Controls.
What are user needs?
Though user needs are on the waterfall diagram, FDA does not actually define the term “user needs” in 21 CFR Part 820.
However, there are a couple references to user needs that help us understand what FDA means by user needs. In CFR 820.30(c), we get the definition for design input, which states:
Each manufacturer shall establish and maintain procedures to ensure that the design requirements relating to a device are appropriate and address the intended use of the device, including the needs of the user and patient.
It’s clear from this definition that the needs of both the end user and patient must be considered. In some cases, the patient will be the user. But in many cases, a healthcare professional like a doctor or nurse will be using the device for the benefit of the patient.
The mention of intended use in this definition is also important. The intended use and indications for use of your device are the basis for determining your user needs.
How do you define your user needs?
The first rule of defining your user needs is: don’t assume anything.
You might be surprised how often companies will assume they understand the user needs without actually soliciting feedback from those users. Remember, internal brainstorming is not a substitute for sitting down with providers and patients to learn exactly what they need from your device.
Defining your user needs is about asking questions and listening. You want to get to the bottom of questions like:
-
What clinical need or problem is the device going to solve?
-
Who is going to use the device?
-
Will the device be used once, or repeatedly?
-
What are the key attributes or features that should be considered?
-
Where and when will the device be used?
-
How will the user interact with the device?
-
What type of procedures will this device be used for?
-
What other products might the device interact with?
Document all the answers to these questions, as they’ll become the list of user needs you use to inform design inputs and eventually validate in the final step of the design control process.
Keep in mind, defining your user needs is required as part of design controls, but it’s not just a box to check as you go through the process. Without a strong focus on the needs of the user, you risk losing sight of what matters most.
How do user needs interact with the rest of design controls?
As the waterfall diagram shows in the zoomed in portion of the graphic below, design inputs are defined by user needs.

A user may need a pacemaker to work for as many years as possible without a battery change, but that’s not a design input. Rather, it’s a user need that will inform design inputs, such as those for battery life and reliability.
It should be clear by now why user needs are found at the top of the diagram. There’s little point in designing and developing a device if you haven’t taken into account the needs of its users.
And as the diagram shows, the final step in the design control process is not a completed medical device. Instead, the final step is validating the device you have made meets the user needs you set down at the very beginning. According to CFR 820.30(g):
Design validation shall ensure that devices conform to defined user needs and intended uses and shall include testing of production units under actual or simulated use conditions.
So design controls don’t just start with user needs; they end with them, as well.
FREE RESOURCE: Click here to download the free eBook of the Ultimate Guide to Design Controls.
Keep user needs at the forefront of your design with Greenlight Guru’s dedicated design control software
One reason user needs may get lost in the shuffle of design controls is that medical device companies simply don’t have a good way to visualize the connections between device requirements. For these companies, their QMS may be paper-based or may rely on generic software that creates opacity in the process.
At Greenlight Guru, we understand the importance of seeing your device’s design in a single, enhanced view. Our purpose-built QMS solution gives MedTech companies an end-to-end system to manage the lifecycle of their product — all in one place.
Greenlight Guru’s dedicated Design Control Software workflow features an innovative and elegant user interface that allows you to clearly see the relationships between user needs, design inputs, risk controls, and components — all in a single, enhanced view.
So, if you’re ready to see your device’s design like never before, then get your free demo of Greenlight Guru today.
Jon Speer is a medical device expert with over 20 years of industry experience. Jon knows the best medical device companies in the world use quality as an accelerator. That's why he created Greenlight Guru to help companies move beyond compliance to True Quality.
Related Posts
Why There is a Need for Multi-Level Design Controls
Why You Need To Stop Treating Risk Management & Design Controls as Checkbox Activities
ALM and Design Controls: Do SaMD and SiMD Manufacturers Need Both?
Get your free eBook
Ultimate Guide to Design Controls











