2024 MedTech Industry Benchmark Report
In our fifth annual medical device benchmark report, we surveyed over 500 medical device professionals to better understand industry-wide trends, shifting priorities and strategies, commercialization challenges, and best practices shaping the MedTech industry.
Download the full reportTop Takeaways
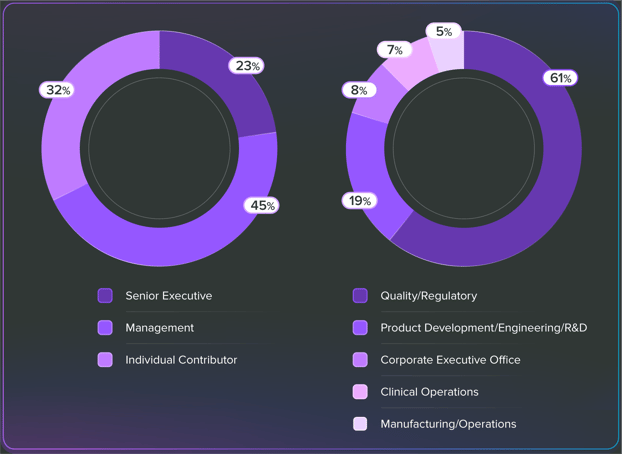
Who took the survey?
Over 500 industry professionals shared their voices, priorities, and biggest hurdles in MedTech for 2024.
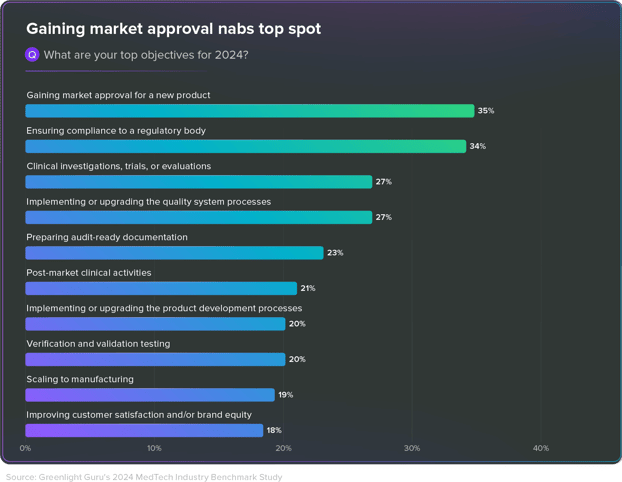
Accelerating New Product Development in 2024
Despite economic uncertainties, optimism for 2024 is high with 86% of respondents expecting revenue growth and market expansion. Gaining market approval has become the top priority for 35% of MedTech professionals, with 45% launching new clinical activities this year.
Growth in 2024 hinges on balancing R&D and operational efficiency, but where does quality fit in? Nearly half (47%) share that enhanced quality management is key to innovation.
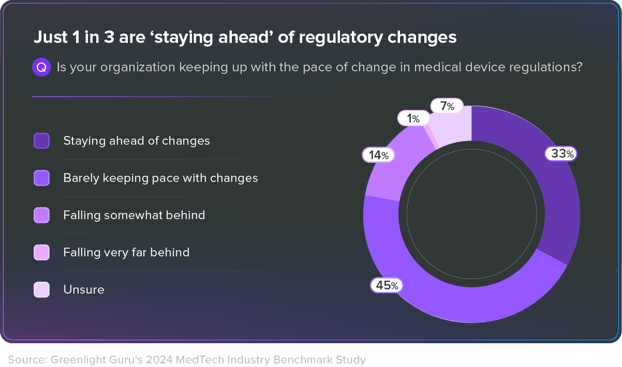
Keeping Up with Compliance
Research reveals: that 33% lead in adapting to evolving regulatory requirements, while 45% struggle to keep up.
Compliance has remained challenging with changing regulations and ineffective quality management systems. Nearly half of organizations are only somewhat confident in their QMS's ability to support their growth in 2024 and beyond.
High-performing companies are prioritizing robust data management practices and technology, with 71% reporting high confidence in demonstrating traceability in an unannounced audit.

Growth Outlook of Clinical Activities
Our research shows a trend of renewed focus on clinical activities. The majority of senior executives (63%) expect their company’s clinical programs to grow over the next two years and 60% of professionals expect their clinical programs are well-equipped to meet 2024 objectives.
Clinical investigations also shifted priority, moving two spots up from the previous year.
Although this growth won’t be without its challenges. 59% say that regulatory agencies are requiring more clinical data than they did previously which also points to a trend of increasing regulatory roadblocks.

Digital Modernization to Drive MedTech Innovation
The medical device industry's adoption of new technologies remains low, with continued underinvestment in tech and data management. 43% of respondents say budgets are flat for technology and digital modernization is not yet a priority.
Despite this, the industry still recognizes the impact and importance of technology's potential benefits, with 71% believing AI adoption is necessary for competitiveness and 55% optimistic about AI's future impact on the MedTech sector.